Classification of Cellular Therapy Products.
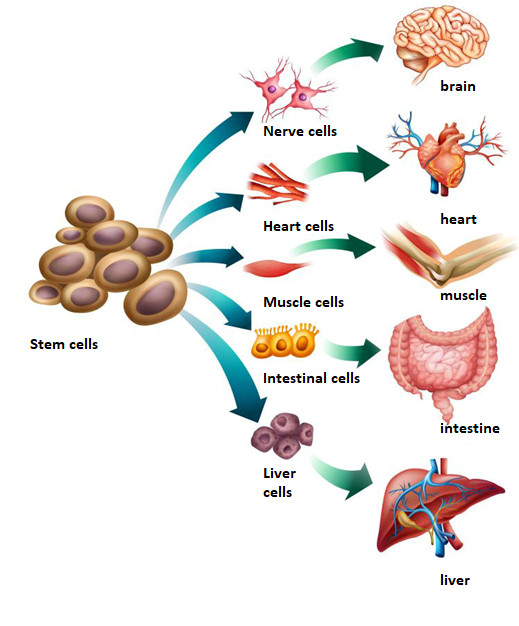
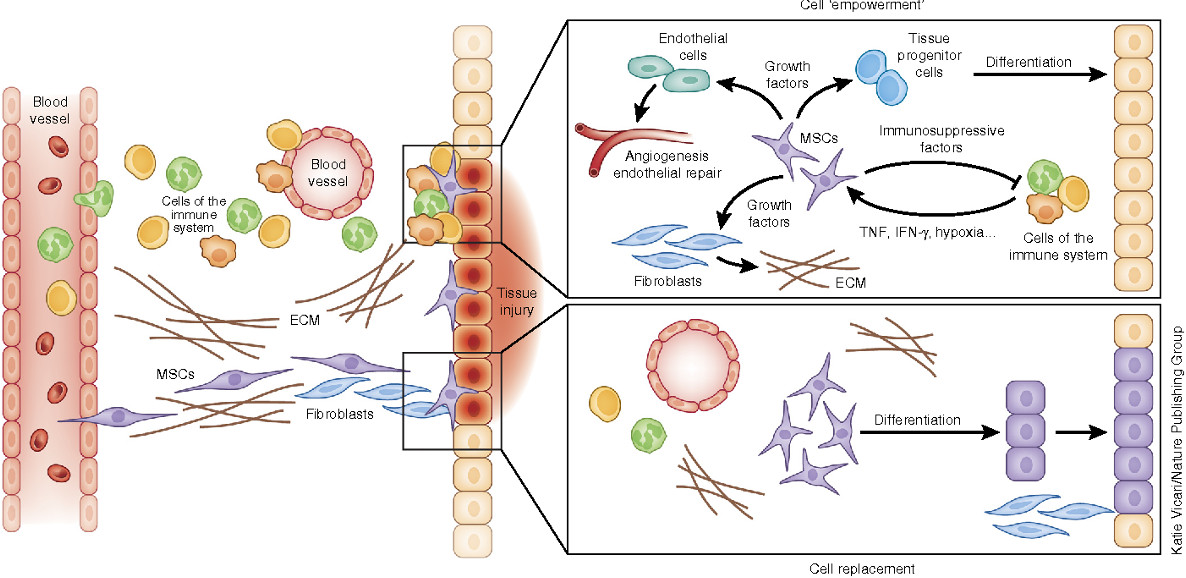
PTC means any preparation that is administered to a human being with purposes similar to medicinal products, and that contains live cells or complex parts of them, whether administered alone or together with matrices/envelopes of synthetic or biological origin. This definition also includes cell populations and products that are part of current clinical practices such as blood transfusions or organ transplants.
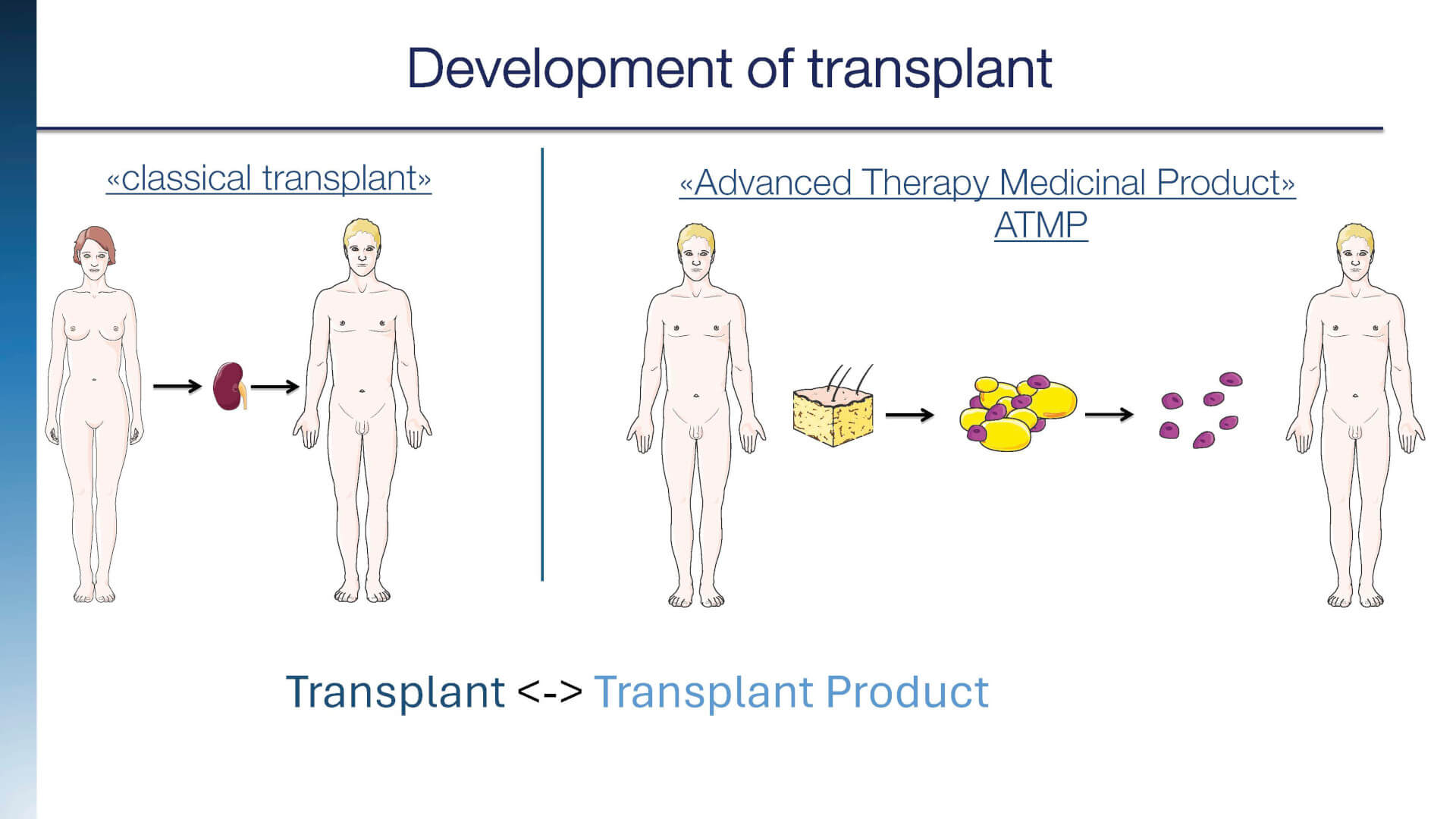
In order to clarify its limits, the criteria currently applied for the identification of a PTC are summarized to distinguish them from those consolidated medical practices in which cell populations are isolated, separated and administered to human subjects either in an autologous manner (same donor-recipient) or allogeneic (different donor-recipient):
I. Included in PTC are those products containing cells or parts of them obtained after "non-minimal" manipulation. Non-minimal manipulation refers to those procedures that alter the genetic, physiological, or biological characteristics of the tissue/organ treated.
II Blood transfusions and plasma derivatives are excluded from the PTC and are regulated by specific legislation. Transplants of vascularized organs or of whole tissue are excluded, and therefore those procedures which involve the removal of all or part of an organ or tissue from donors or cadavers and their transplantation in a short time into a recipient subject, after minimal ex vivo manipulation. Minimal manipulation means a procedure that does not alter the genetic, physiological, or biological characteristics of the tissue/organ treated.